Mesh|EX®
a Skin Graft Meshing Device
The Perfect Graft Deserves the Perfect Mesh.
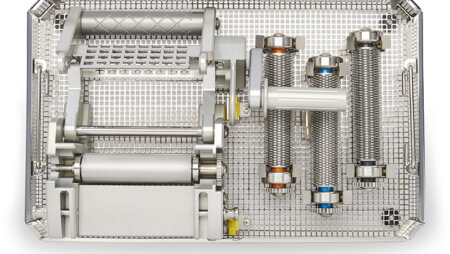
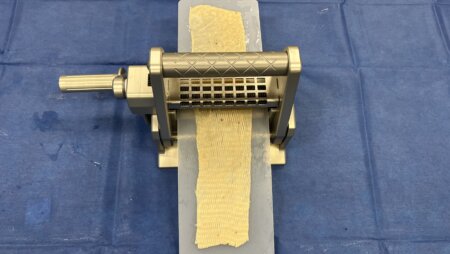
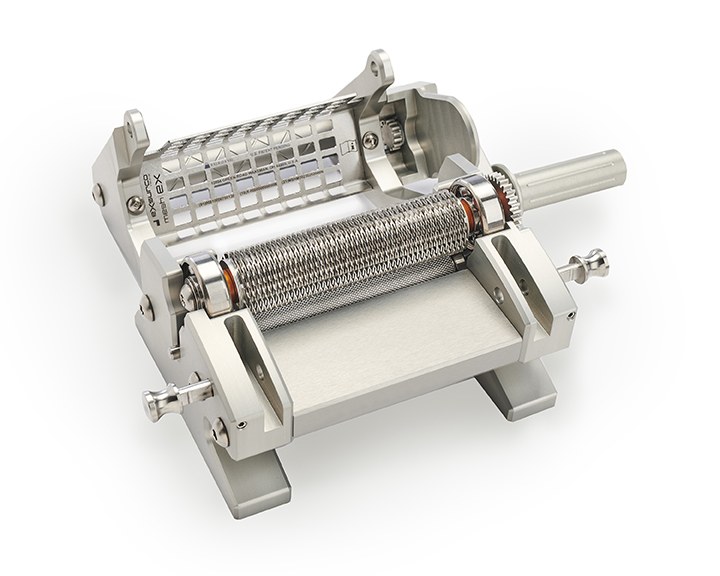
The Exsurco Mesh|EX is a mechanically powered skin graft meshing device. Mesh|EX is capable of creating three unique mesh ratios on varying thickness of skin grafts. A separate cutting roller for each mesh ratio is included for use depending on tissue graft size needs.
Mesh|EX was designed to deliver consistent and precisely meshed skin grafts.
Usability is designed into the Mesh|EX to help drive clinical outcomes, including:
- Wide graft meshing capabilities (up to 4.25")
- Three easily exchangeable cut ratio rollers
- Continuous crank handle for smooth and fast operation





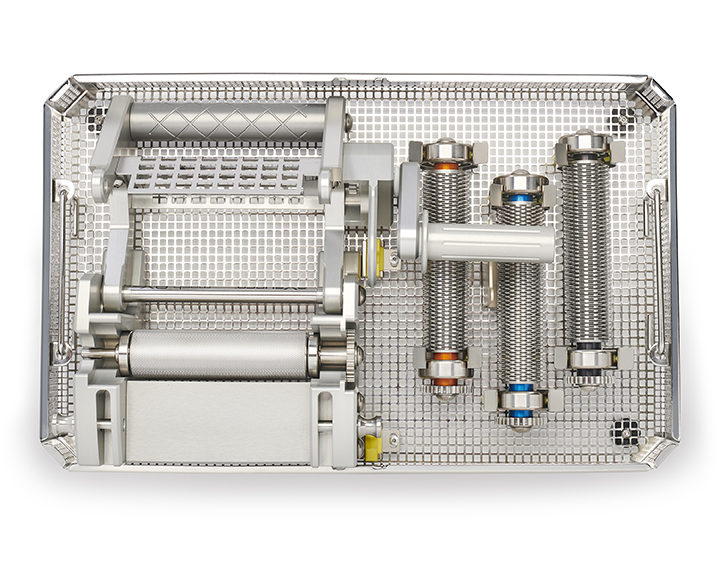



 Mesh|EX Specifications:
Mesh|EX Specifications: