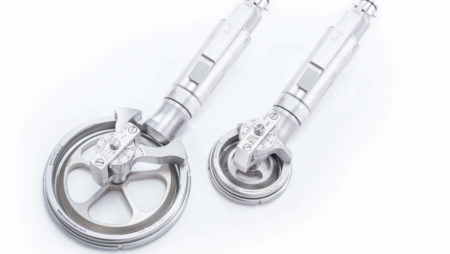
The Amalgatome® SD is a Skin Grafting and Wound Debridement Device
One Device. Multiple Capabilities.
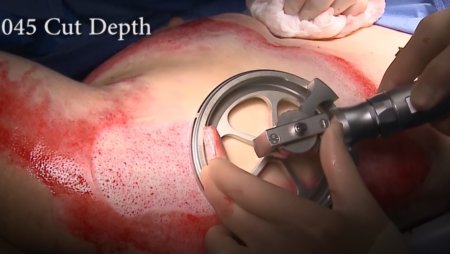
Engineered to deliver the best possible outcomes for burn and trauma patients, the Amalgatome® SD represents a decisive leap forward in skin grafting and wound debridement technology.
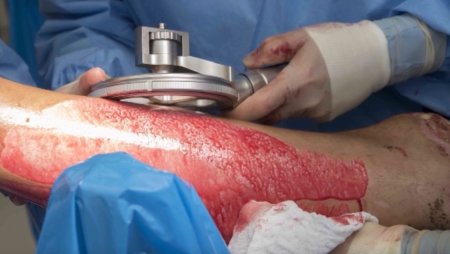
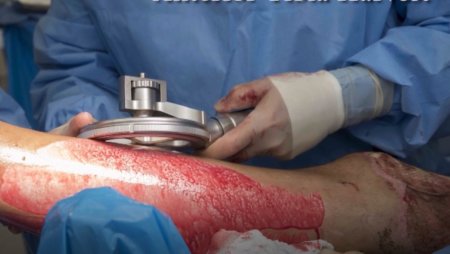
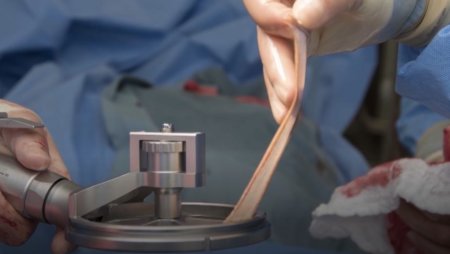
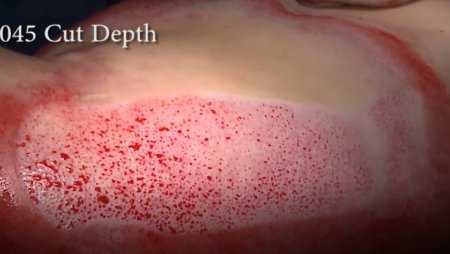
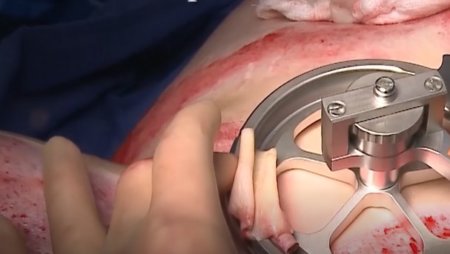
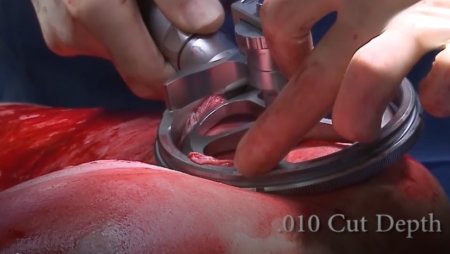
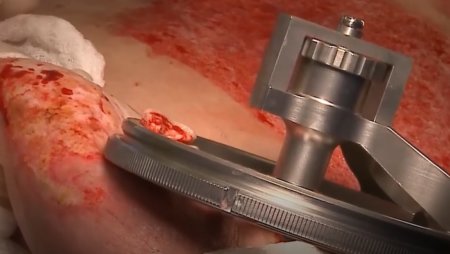
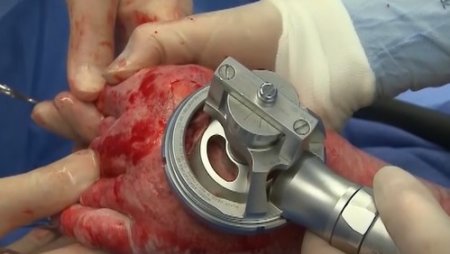
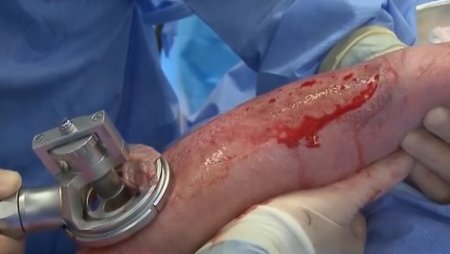
The pneumatically powered Amalgatome SD provides effective wound debridement, including removal of necrotic tissue and eschar, as well as being able to produce uniform, consistent skin grafts in thickness ranging from 0.005" to 0.045" in increments of 0.001 inches. All combined into one device.
Studies on file demonstrating the Amalgatome SD:
- Provides superior graft quality
- More consistent thickness for excised necrosis tissue and skin grafts
- A better relationship between the set depth of excision and actual depth
- Overall ease of use and assembly compared to standard technology

 Amalgatome SD Specifications:
Amalgatome SD Specifications: